By Lindsey Schmidt, Eco-Journalism and Blogging Intern
As Michigan residents, we are constantly hearing about PFAS and the dangers they pose to the environment and our health. Michigan has been deemed a “hotspot” for PFAS, but is this really an accurate statement? And what is PFAS, anyway? If you’re looking to find an answer to either of those questions, hopefully this article can help clear up your confusion.
What is PFAS?
The first PFAS, or polyfluoroalkyl substance, was developed by Roy J. Plunkett, a researcher at DuPont’s Jackson Laboratory. He developed polytetrafluorethylene (PTFE), which was introduced in the 1940s under the trade name Teflon. Beginning in the 1950s, DuPont and 3M became the leading manufacturers of PFAS. In 2006, DuPont promised to discontinue the manufacture and distribution of perfluorooctanoic acid (PFOA), and the company was able to achieve their goal by 2015. 3M has also agreed to phase out the use of PFAS and has promised to completely discontinue their use of the chemicals by 2025.
PFAS encompasses both per- and poly-fluoroalkyl substances and is a large group composed of thousands of different chemicals. Perfluoroalkyl substances contain a long chain of carbon-carbon bonds in which each carbon in the chain is fully saturated with fluorine. Polyfluoroalkyl substances are similar in structure, but they are only mostly saturated with fluorine; the carbon chains still contain some carbon-hydrogen bonds.
Figure 1: The structure of perfluorooctanoic acid (PFOA), a perfluoroalkyl substance.
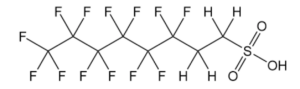
Figure 2: The structure of 6:2 fluorotelomer sulfonate (6:2FTS), a polyfluoroalkyl substance.
Because carbon-fluorine bonds are incredibly strong, these chemicals are heat, oil, water, grease, and stain resistant. As such, they are used to make a variety of items, including non-stick frying pans, waterproof rain jackets, flame retardant sofas and carpets, food packaging, and makeup. Unfortunately, the strength of the bonds in PFAS also means that these chemicals are highly resistant to breakdown. Substances that are resistant to breakdown are known as “forever chemicals” and can accumulate in our bodies and in the ecosystem with potentially dangerous consequences.
Where can PFAS be found?
PFAS can leak into our natural water supply via household sewage systems and landfill runoff. Since its development, PFAS have steadily been building up in both freshwater and saltwater systems. PFAS can be found wherever the products mentioned above are being used; in other words, PFAS can be found pretty much anywhere. As Michiganders, you may have heard that Michigan is a hotspot for PFAS. However, this statement is not entirely accurate. PFAS can and will be found anywhere if and when people bother to look for it. Michigan has identified more PFAS sites than any other state in the US because we have a comprehensive action team that actively looks for PFAS sites. Just as much PFAS would be found in other states as well if they were to also implement comprehensive PFAS response teams.
After accumulating in the water supply, PFAS can make its way into humans and animals via direct ingestion of water and consumption of contaminated seafood. The Center for Disease Control and Prevention (CDC), using data from the National Health and Nutrition Examination Survey (NHANES), found that PFAS were present in the blood of 97% of Americans. Four particular PFAS were found in the blood of nearly all people tested: PFOA, perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA).
In addition to building up in both freshwater and saltwater wherever PFAS products are used, PFAS is able to reach remote corners of the world via the water cycle. Because PFAS are forever chemicals, they can cycle throughout the atmosphere with water and reach remote locations in rain or snow, with PFAS even being detected in snow and algae in Antarctica. PFAS’s persistent nature and ability to survive the water cycle indicates that PFAS contamination is, as of now, largely irreversible.
The Department of Environment, Great Lakes, and Energy (EGLE) has a variety of maps available that pinpoint PFAS contamination sites throughout Michigan. There are two PFAS sites in Grand Haven: the site of the former J.B. Sims Generating Station, located on Harbor Island, and Challenge Machinery Company. The proximity of these sites to the Grand River means they pose a risk to the Lower Grand River Watershed and, subsequently, Lake Michigan. Harbor Island’s proximity to Spring Lake also indicates that these two PFAS contamination sites could impact the Spring Lake/Norris Creek Watershed. As well as a PFAS contamination site map, EGLE provides a map of surface water sampling sites. Recent data show the PFAS contamination levels of seven sampling sites in Grand Haven, all along the Grand River. Each point contains the contamination levels of several different PFAS molecules, all recorded in parts per trillion (ppt). The Environmental Protection Agency (EPA) recommends that the total PFAS contamination level of drinking water should be no higher than 70 ppt. According to the most recent data, there are no sites in Grand Haven with a PFAS concentration that exceed 70 ppt; in 2018, the nearby Robinson Elementary School’s water supply tested at 144 ppt, but that has since been rectified.
What are the health risks associated with PFAS?
Studies of laboratory animals have shown that large amounts of PFAS exposure can affect growth and development, reproduction, thyroid function, the immune system, and the liver in both humans and wildlife. Additionally, PFAS is thought to increase the risk of certain cancers and kidney disease, increase bad cholesterol, disrupt reproductive functioning, and decrease vaccine response. A study of East Greenland polar bears provided evidence for the disruption of reproductive functioning, as PFAS negatively impacted their hormone systems.
Luckily, PFAS is only shown to have major impacts on health after significant exposure to the chemicals, and we are unlikely to experience such drastic effects as a result of our relatively low, day-to-day exposure to PFAS. Additionally, since companies such as DuPont and 3M started discontinuing the use of PFAS, levels of PFOS and PFOA in the blood have decreased. This does not mean PFAS is no longer an issue; unfortunately, new chemicals are being developed to replace PFOS and PFOA, and these new chemicals could still pose a threat to the environment and our health. Furthermore, the cessation of PFOS and PFOA development does not undo the damage that has already been done. PFAS will continue to be a problem until we are able to come up with a way to remove it from our water supply which, as of now, has not been accomplished.